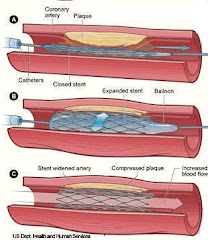
We hope that this blog has allowed you to follow the development of percutaneous coronary intervention, which began in 1977 when Andreas Gruntzig first introduced balloon angioplasty and which has progressed to second/third-generation drug eluting stents and biodegradable stents. By looking at this path, one can appreciate the many difficulties researchers have faced trying to treat obstructive coronary artery disease., Yet, it is encouraging that recent technological gains with coronary stents do appear to prevent restenosis of the artery while limiting serious side effects.
On multiple occasions, this blog has shown when one problem is solved by a new technology, unfortunately another one seems to be created. Balloon angioplasty was first developed for the management of coronary stenosis. Even though this was a major breakthrough, it had two major drawbacks, a high risk of immediate dissection of the artery followed by a risk of restenosis occurring later in a little over one half of the patients. To solve the problem of immediate dissection, balloon expandable bare metal stents were developed in 1987 to provide support to the artery. Restenosis also showed reduction with bare metal stents, with the risk of restenosis around 30% in patients. In the early 21st century, drug-eluting eluting stents were designed to inhibit restenosis by releasing pharmacological agents (typically anti-proliferative compounds which induced cell cycle arrest in G1 phase) to block vascular smooth muscle cell proliferation. Many of the articles reviewed in this blog have shown that these drug eluting stents have been able to prevent neointimal hyperplasia and arterial remodeling, which are the two main processes that lead to restenosis. Restenosis rates had dropped to below 10% in patients who had received a drug-eluting stent.
Although drug-eluting stents proved to reduce restenosis, long term followup studies began to suggest that they might be associated with a higher risk for stent thrombosis as compared with bare metal stents. Some of the drugs used in the stents such as rapamycin and paclitaxel have been shown to induce the expression of tissue factor, an important part of the initiation of coagulation and thrombus formation. Also, the polymers used to hold and release the antiproliferative drugs occasionally caused local allergic reaction resulting in chronic inflammation which predisposed to thrombosis.
The current health care debate, therefore, is whether these drug-eluting stents are more advantageous than bare metal stents or if the risk of thrombosis is too high and do not outweigh the benefits. At this point, it is our opinion that drug-eluting stents do provide more benefit than risk and it is a sustainable technology. However the thrombosis risk deserves continued close surveillance. It is also recommended that patients who receive drug-eluting stents take aspirin and Plavix for at least a year after the procedure to lessen the chance of clotting. Still, it is the hope that future coronary stents will be further improved so that these deleterious side effects will be eliminated and that the duration of dual antiplatelet agents, were there incumbent risk of serious bleeding, can be limited.
For the future, when developing new drug-eluting stents, researchers need to attempt to develop stents that can eliminate or have a significantly reduced incidence of restenosis. Researchers should also concentrate on developing stent materials/ polymers that do not cause hypersensitivity reactions and simultaneously reduce the risk of thrombosis. Multiple technologies, including novel antiproliferative agents, polymers, and stent designs, are currently being researched and designed to be used in second and third generation stents. For example, the SPIRIT trial investigated the use of a newer ‘limus’, everolimus, believed to be a more effective antiproliferative agent, embedded on a less toxic polymer matrix, mounted on a thinner strut, more maneuverable stent. Also, bioabsorbable stents have been gaining a lot of interest. They are thought to represent an alternative revascularization modality that would address the short-term need for a vessel scaffolding but avoid the complications of thrombosis and inflammation seen when a foreign body is left within a vessel. The bioabsorbable magnesium stents are promising in that magnesium is expect to have beneficial effects by acting as a systemic and coronary vasodilator. Initial results of the Absorbable Metal Stent (AMS) from Biotronik have been favorable. This stent appears to reduce intimal proliferation, to have no stent-related adverse effects, and to have no prothrombotic effects. Although these new technologies appear very promising, more and larger clinical studies are needed before they can be viable options for patients with coronary artery disease. We have learned our lesson with the (too)rapid acceptance of DES technology- new technologies often bring new, unexpected problems that can not be identified in initial, small patient trials.
Although much controversy has surrounded drug-eluting stents, we believe that they are currently the best viable for the percutaneous treatment of obstructive coronary artery disease. As for the immediate future, second and third generation stents appear to continue to lower restenosis rates while reducing potentially dangerous side effects. But stent polymer and drug technology will continue to progress exponentially. In the future, we expect that we will have multiple different stents allowing us to tailor the stent and the drug to the patient's disease state. For instance, diabetics may require a more potent antiproliferative than non diabetics. Older, more debilitated patients with a higher risk of bleeding and an inability to take dual antiplatelets will need a stent with less thrombosis potential. The future is always difficult to predict but it is quite apparent is that our third generation stents are not the end, rather they are just the beginning in the evolution of a very promising technology.